อ้างอิง:
ข้อความเดิมเขียนโดยคุณ ekkewbabay
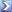
In a building the ventilation system operates when the concentration of carbon dioxide reaches a certain level.Suppose that when the ventilation system operates,the cubic feet of carbon dioxide,X,in an 8000 cu ft room depends on time t (in minutes) according to x=4.8+11.2(e^(-t/4))
a).Find the initial amount of carbon dioxide in the room,and find the concentration of carbon dioxide at this time.
b).How long does it take to have a concentration of 0.07% carbon dioxide?
c).The steady-state or equilibrium concentration is what would result if the ventilation system were left on indefinitely. By using large values for t ,determine the steady-state concentration.
|
The amount in cubic feet of carbon dioxide is a function of time, given by
\[
X(t) = 4.8 + 11.2 \cdot e^{-t/4}
\]
(a) Initially, $t=0$. We have $X(0) = 4.8 + 11.2 \cdot e^{0} = 4.8 +11.2 = 16.0$ cubic feet. This is, in concentration, $0.2 \%$.
(b) The concentration of $0.07 \%$ carbon dioxide is precisely $0.0007 \times 8000 = 5.6$ cubic feet. To determine $t$, we solve
\[
X(t) = 4.8 + 11.2 \cdot e^{-t/4} = 5.6
\]
so $t \approx 10.56$ minutes.
(c) As the machine goes on indefinitely, the amount of carbon dioxide will converge to
\[
\lim_{t \rightarrow \infty} 4.8 + 11.2 \cdot e^{-t/4} = 4.8
\]
which is, in concentration, $0.06 \%$.
Please let me know if any of above is not clear.
